|
SUGAR - SUCROSE
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
HOME | BIOLOGY | FILMS | GEOGRAPHY | HISTORY | INDEX | INVESTORS | MUSIC | SOLAR BOATS | SPORT |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Either known as cane sugar when made out of sugar cane or as beet sugar when made out of sugar beets. Don´t be confused both are the absolute identical chemical compound. Sugar is one of the purest commercialy distributed organic "chemicals" produced in millions of tons. There is no need to buy analytical grade sugar for crystal growing experiments, its only something for scientists or if you have to much money. Just take the regular sugar from the next supermarket - watch for sales (sugar can not rot) !
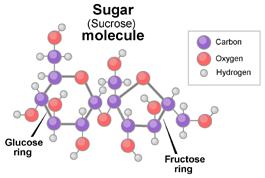
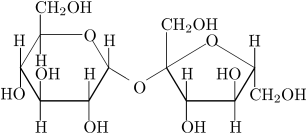
The white stuff we know as sugar is sucrose, a molecule composed of 12 atoms of carbon, 22 atoms of hydrogen, and 11 atoms of oxygen (C12H22O11). Like all compounds made from these three elements, sugar is a carbohydrate. It’s found naturally in most plants, but especially in sugarcane and sugar beets—hence their names
In general use, "sugar" is taken to mean sucrose, also called "table sugar" or saccharose, a disaccharide which is a white crystalline solid. It is the most commonly used sugar for altering the flavor and properties (such as mouthfeel, preservation, and texture) of beverages and food. Table sugar is commercially extracted from either sugar cane or sugar beet. The word sugar originates from the Sanskrit word sharkara (शर्करा) which means "sugar" or "pebble."
The "simple" sugars, or monosaccharides, such as glucose, are a store of energy which is used by biological cells. A sugar is denoted by any word on the ingredient list that ends with "ose".
For information on the other sugars, see monosaccharide and disaccharide. In precise culinary terms, sugar is a type of food associated with one of the primary taste sensations, that of sweetness.
Physical and Crystallographical Properties
Sugar price reaches 25-year high - Friday, 03/02/2006 http://www.abc.net.au/
The price of sugar has broken through the 19 US cents per pound barrier to reach its highest price in 25 years.
The commodity soared by nearly a cent to close at 19.15 in international trade. Ian White from Queensland Sugar says prices are spiking because it is the fourth year of a world sugar supply deficit. He says it is a very positive sign for the price of Australian sugar in the next 12 to 18 months.
"The value of sugar just in the raw without taking costs off and so forth is up over $500 a tonne and obviously that will translate into very good pool prices, certainly in the high 400s," he said.
Sugar was first produced in India. Alexander the Great's companions reported seeing "honey produced without the intervention of bees" and it remained exotic in Europe until the Arabs started cultivating it in Sicily and Spain. Only after the Crusades it began to rival honey as the sweetener in Europe. The Spanish began cultivating sugar cane in the West Indies in 1506, and in Cuba in 1523. It was first cultivated in Brazil 1532 by the Portuguese. [1]
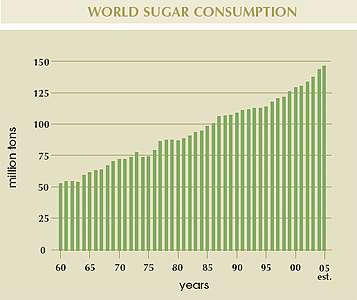
Table sugar or sucrose is extracted from plant sources. The most important two sugar crops are sugarcane (Saccharum spp.) and sugar beets (Beta vulgaris), in which sugar can account for 12%–20% of the plant's dry weight. Some minor commercial sugar crops include the date palm (Phoenix dactylifera), sorghum (Sorghum vulgare), and the sugar maple (Acer saccharum). In the financial year 2001/2002, 134.1 million tonnes of sugar were produced worldwide.
The major cane sugar producing countries are countries with warm climates, such as Brazil, India, China and Australia (in descending order). In 2001/2002 there was over twice as much sugar produced in developing countries as in developed countries. The greatest quantity of sugar is produced in Latin America, the United States and the Caribbean nations, and in the Far East.
The sugar beet regions are in cooler climates: North West and Eastern Europe, Northern Japan, plus some areas in the United States including California. The beet growing season ends with the start of harvesting around September. Harvesting and processing continues until March in some cases. The duration of harvesting and processing is influenced by the availability of processing plant capacity, and weather - harvested beet can be laid up until processed but frost damaged beet becomes effectively unprocessable.
The world's second largest sugar exporter is the EU. The Common Agricultural Policy of the EU sets maximum quotas for members production to match supply and demand, and a price. Excess production quota is exported (approx 5 million tonnes in 2003). Part of this is "quota" sugar which is subsidised from industry levies, the remainder (approx half) is "C quota" sugar which is sold at market price without subsidy. These subsidies and a high import tariff make it difficult for other countries to export to the EU states, or compete with it on world markets. The U.S. sets high sugar prices to support its producers with the effect that many sugar consumers have switched to corn syrup (beverage manufacturers) or moved out of the country (candy makers).
The sugar market is also under attack from the cheap prices of glucose syrups produced from wheat and corn (maize). In combination with artificial sweeteners, drink manufacturers can produce very low cost products.
Cane
The harvested vegetable material is crushed, and the juice is collected and filtered. The liquid is then treated (often with lime) to remove impurities, this is then neutralized with sulfur dioxide. The juice is then boiled, sediment settles to the bottom and can be dredged out, scum rises to the surface and this is skimmed off. The heat is removed and the liquid crystallises, usually while being stirred, to produce sugar crystals. It is usual to remove the uncrystallised syrup with a centrifuge. The resultant sugar is then either sold as is for use or processed further to produce lighter grades. This processing may be carried out in another factory in another country. Beet
The washed beet is sliced, and the sugar extracted with hot water in a 'diffuser'. Impurities are precipitated with an alkaline solution "milk of lime" and carbon dioxide from the lime kiln. After filtration the juice is concentrated by evaporation to a content of about 70% solids. The sugar is extracted by controlled crystallisation. The sugar crystals are removed by a centrifuge and the liquid recycled in the crystalliser stages. Liquid from which no more sugar can be economically removed is lost from the process as molasses and used in cattle food.
The white sugar produced is sieved into different grades for selling. Cane versus Beet
There is little perceptible difference between sugar produced from beet and that from cane. Testing for impurities can distinguish the two, and these have been developed to reduce fraudulent abuse of EU subsidies, and also aid detection of adulteration of fruit juice.
The residues of sugar production differ substantially and from place to place. While cane molasses can be used as an ingredient, molasses from sugar beet is unpalatable and generally used for industrial fermentation or as animal feedstuff. Cane and beet pulp can be burnt for fuel, but beet pulp is generally dried, pelleted and used as an animal feedstuff.
|
|
|
PRODUCTION |
EXPORTS |
POPULATION |
PER CAPITA CONSUMPTION KGS |
|
Brazil |
29.151 |
17.757 [1] |
181 |
54 |
|
EU |
21.381 |
6.210 [2] |
461 |
39 |
|
India |
13.587 |
0 [-] |
1,086 |
17 |
|
China |
10.652 |
0.109 [17] |
1,324 |
9 |
|
USA |
7.362 |
0.173 [14] |
297 |
29 |
|
Mexico |
5.762 |
0.054 [19] |
105 |
52 |
|
Australia |
5.516 |
4.465 [3] |
20 |
46 |
|
Thailand |
5.326 |
3.361 [4] |
64 |
35 |
|
SADC |
5.173 |
1.168 [7] |
163 |
21 |
|
Pakistan |
3.429 |
0.109 [17] |
154 |
23 |
Despite the rise in world raw sugar prices during the past year, preferential prices in Europe and the United States prices remain at a considerable premium. The continued strength of the Euro has further increased ACP (African, Caribbean and Pacific) and SPS (Special Protocol Sugar) prices in US$ terms.
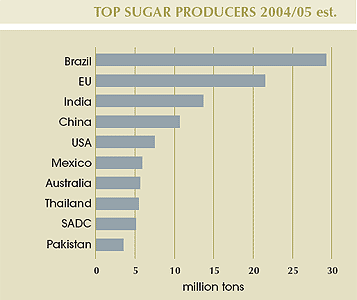
Global sugar production in 2004/05 is estimated at 144 million tons, 75% of which is produced by the world’s top ten sugar producers. With the enlargement of the EU to 25 countries, 21 of which produce sugar, total production in this region compared to last year has increased by about 5 million tons to approximately 21 million tons.
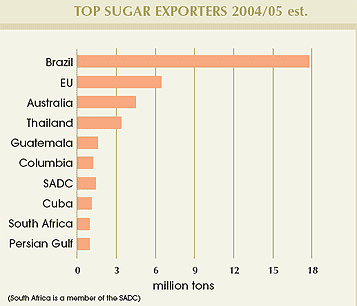
Approximately 70% of total world sugar production is consumed in the country of origin with the balance traded on global markets. As the world’s largest sugar producer, Brazil remains the world’s dominant sugar exporter.
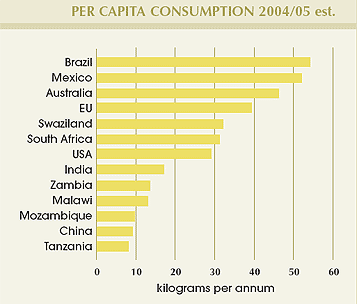
For the second year in succession, per capita sugar consumption in China has increased, due primarily to the recent upturn in that country’s economy. Consumption trends in other countries, however, are similar to previous years.
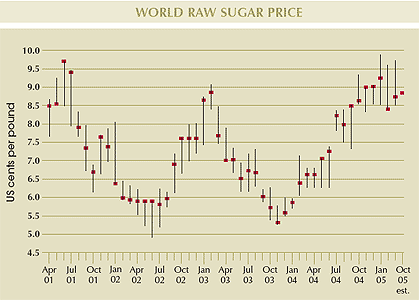
As a result of the current global sugar production deficit, world raw sugar prices, whilst remaining volatile throughout the season, saw an upward adjustment during the year, rising from the nearby futures price of US7.15 cents/lb in March 2004 to over US9.00 cents/lb by March 2005.
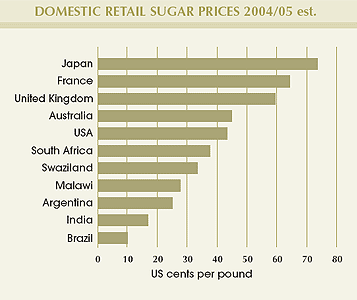
Domestic sugar prices in the South African Customs Union remain substantially below those of some developed nations. The last (2005) independent survey of international sugar production costs revealed that Southern African countries remain amongst the world's lowest-cost sugar producers.
Solar Cola sponsor this website
We are looking for distributors in America, Australia, Canada, Europe, and Japan. The state of the Cola market globally and in the UK is ripe for a fresh quality brand, with excellent potential for growth. According to ResearchandMarkets.com the UK drinks market is worth an estimated £53.5 billion, representing a 7% share of total consumer spending. The global soft drinks market is roughly the same percentage of total consumer spending for developed countries.
Prospective investors in our company should consult their own independent investment advisers, and please note this information is provided for general guidance only. It is not a prospectus, but is provided in response to the number of requests we have received asking for more information
GROWING SUGAR CRYSTALS FROM WATER SOLUTION
There are two simple
basic methods to grow crystals from a solution:-
-
The Evaporation Method
-
The Slowly Cooling Method
Using the evaporation method you simply evaporate the solvent (in this case water) of your saturated solution to get crystals. Its quite simple but may take a long time. If the solubility is low you may wait a very long time to get nicely sized crystals. Fortunately in the case of sugar the solubility is very high.
Using the slowly cooling method you produce a
hot saturated solution cool it down
slowly to get the crystals.
The trick here is to let it cool down really
slowly. The slower a solution cools down,
the bigger and finer
the crystals will be. Unfortunately, this method
does
not work with substances which do not change
solubility greatly with rising temperature (like
regular table salt) or which mixes solubility goes
down with rising temperatures. Fortunately the
solubility of sugar rises greatly when the
temperature goes up.
The good thing is this method is quick you will
get nice sized crystals within several hours,
but try it over days for comparison
purposes.
The Evaporation Method
Dissolve per 100 grams of water 230 grams of sugar heat up the solution until it boils and becomes clear. Then, if you want, filter trough a regular coffee filter with paper. But filtering is not absolutely necessary. The solution may have a slight yellow hue. For heating up the solution use a cooking pot or a vessel made from heat resistant glass (pyrex), as for example replacement jars for electric coffee machines. You may also use the microwave but of course only use vessels which are suitable for this (no metal or metal parts !).

Sugar beet factory Idaho USA (painting)
To grow the crystals you can use any kind of glass or plastic container with a wide open mouth. For example preservation glasses etc. You should produce at least about 500 ml of solution better around 1000 ml (or a quart). For a method how to calculate a specific volume of growing solution see the "Calculating a Solution" part down below.
After the covered solution has cooled down so
after two or three days there should be some
sugar crystals at the bottom of the jar. If not
throw in some little grains of sugar. Let the
solution stay alone and covered for about a
week.
If you got no crystals on the bottom, yet even
after throwing in some sugar grains your
solution can not be saturated and won't work.
This may happen either because you made a
mistake with the amounts of water and sugar used
or your room temperature is well above 20 °C
(app. 70 °F).
To avoid mistakes in the amounts of water and
sugar used, use an electronic kitchen scale
which
should have at least a resolution of 2 grams
(better 1 gm) most can be switched from oz./lbs
to metric, metric is easier to calculate. Also
weigh the water as its much more accurate as
measuring the volume.
If your room temperature is well above 20 °C
you have to adjust the initial recipe. If you
look at the solubility table down below the
solubility of sugar at 20 °C is 203 gms per 100
gms of water. In the recipe we took 230 gms (27
gms added as security gap). If you work for
example at a room temperature of 30 °C you
adjust the recipe to 250 gms per 100 gms of
water (again app. 30 gms as security gap).
Okay everything worked fine, there are some
crystals at the bottom and the solution rested
for a week. Now pour the solution in your final
freshly cleaned growing vessel. You may filter
but its not absolutely necessary and filtering
the viscose solution may take for ever.
Now you need a seed crystal, usually you will
find at the bottom nicely sized sugar crystals
already suitable for this purpose or you may use
a bought candy sugar crystal (that's cheating
!). Dry up the crystal with some paper towel and
fix it with a slip knot to a thread of
"invisible sewing thread" which is a
thin clear thread of nylon or use very thin
fishing line. Don't use regular threads made
out of cotton etc. as they work like a wick and
are easily visible in the ready crystal.
Fix the thread to a piece of wood or a pencil for
example so that the crystal suspends somehow
above 2 - 3 cm (about one inch) the bottom of
your growing vessel but well below the surface
of the solution. Before doing that rinse the
crystal on the thread shortly in cold water.
The growing vessel must stay open to allow the
water to evaporate but you may cover it with a
thin paper towel (most paper towels are multi
layer so you may split them) to prevent flies,
wasps, dust etc. from falling into the solution.
As the water evaporates your seed crystal will
grow. This will not work if you are in a very humid
climate or if your room temperature changes and
goes up.
As evaporation goes on there may grow additional
crystals on the bottom of the vessel on the
thread or on the sides. They grow on the cost of
your desired main crystal. If so, pour the
solution in a freshly cleaned other vessel rinse
the crystal and the thread shortly in cold water
(remove additional crystals which may have
formed on the thread) and go on with
evaporation.

Sugar factory Brooklyn USA
The Slowly Cooling Method
If you produced the saturated solution for the evaporation method and found some crystals at the bottom you already used the slowly cooling method to produce crystals ! To get bigger and better ones you just have to add more sugar (larger "security gap") and take care that the solution cools down very slowly.
A good basic recipe is to add 230 to 300 gms of
sugar to 100 gms of water, heat up until the
solution boils and gets clear. You may filter if
you want but its not absolutely necessary. Pour
the solution in your final growing vessel and
close it tightly. Take care that the the
solution cools down very slowly by insulating it
and also avoid any moving, shaking or vibration
of the solution.
You may give the crystals a better surface to
grow, a matrix, if you put in a piece of rock,
or have it suspended on a thread, or use a metal
paper clip on a thread.
It takes a few hours to days, depending on how
much solution you take and how good your
insulation is, until the solution has cooled
down to room temperature and the crystals are
ready.
They main problems you may have with the slowly
cooling method is that the solution does not
cool down slowly enough, which results in small
crystals or your solution has cooled down but
there are no crystals at all !
What happened ? Super-saturation ! Your solution
contains much more sugar as "allowed"
since there have not formed any seed crystals
spontaneously. If the solution is disturbed or
you throw in some little sugar grains
crystallisation starts immediately.
Since the growing velocity of sugar crystals is
small, solutions are often slightly
supersaturated when they have cooled down and so
the crystals still grow a little while even if
the temperature does not change anymore. So
allow the crystals some extra time.
The Trick with the Thermal Ballast
As more volume of solution you have as longer it will take to cool down. The reason is that the surface of your vessel is growing by power of two but your volume and so the amount of heat energy is growing by power of three if you enlarge the dimension of your vessel. The heat loss depends on the surface area available. To give you a simple example lets say we have a cubic container of 1 inch, so the surface is 6 square inches and the volume one cubic inch. Now lets take a container of 10 inches size the volume will be 10*10*10 = 1000 cubic inches but the surface only 6*10*10 = 600 inches. With the small container you have a volume/surface ratio of 1 to 6 with the big one a ratio of 10 to 6 and its a good guess that cooling down takes ten times longer. So that was the physical principle !
However who wants to handle gigantic amounts of
solution just to grow some little crystals ?
Nobody told you that all of the volume must be
solution! Only a small amount of solution and a
large amount of water will have the same effect
if you keep them separated.
The pictures down below explain that to you.
Just heat up a big cooking pot of water and
separately prepare some solution. Then put the
container with the hot solution in your cooking
pot with hot boiling water. Take care that both
are closing tightly. If you put your cooking pot
in a big box with insulation material, like
styrofoam, cotton, rock wool, saw dust etc. you
get a real slow cooling down of several days up
to over a week. If you use an electronic
thermometer you can watch the temperature
falling.
Calculating a Solution
You often will have the problem that you need a specific volume of solution to fill a growing vessel efficiently. Lets take for example you want to use the slowly cooling method, have a 1000 ml jar and you want to have about 800 mls of solution which contains 260 gms of sugar per 100 gms of water.
The specific gravity of sugar is 1.587,
so 260 gms have a volume of 260/1.587 = 163.8 ml
+ the 100 gms of water (which are almost exactly
100 mls)
you get a volume of 263.8 mls.
To fill up 800 ml you need 800/263.8= 3.03 times your basic recipe.
That is 3.03*100 gms = 303 gms of water and
3.03*260 gms = 787.8 gms of sugar.
If you take 300 gms of water and 790 gms of sugar you will also be fine.
If you do not want to calculate by hand you can use this little calculator.
Conclusion
If you follow this guide should learn something about growing sugar crystals and the basics of solution growth. The principles are the same growing other crystals.
This website is Copyright © 1999 & 2007 NJK. The bird logo and name Solar Navigator and Solar Cola are trademarks. All rights reserved. All other trademarks are hereby acknowledged. Max Energy Limited is an educational charity.
AUTOMOTIVE | BLUEBIRD | ELECTRIC CARS | ELECTRIC CYCLES | SOLAR CARS