|
GLUCOSE |
||||||||||||||||||||||||||
|
HOME BIOLOGY CREW GEOGRAPHY HISTORY INDEX MUSIC THE BOAT SOLAR CELLS |
||||||||||||||||||||||||||
|
Glucose (Glc), a monosaccharide, is one of the most important carbohydrates. The cell uses it as a source of energy and metabolic intermediate. Glucose is one of the main products of photosynthesis and starts cellular respiration. The natural form (D-glucose) is also referred to as dextrose, especially in the food industry. This article deals with the D-form of glucose (see Isomers-section below)
Structure
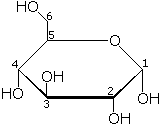
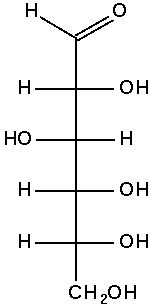
Glucose contains six carbon atoms and an aldehyde group and is therefore referred to as an aldohexose. The glucose molecule can exist in an open-chain (acyclic) and ring (cyclic) form, the latter being the result of an intramolecular reaction between the aldehyde C atom and the C-5 hydroxyl group to form an intramolecular hemiacetal. In water solution both forms are in equilibrium, and at pH 7 the cyclic one is the predominant. As the ring contains 5 carbon and one oxygen atoms, which resembles the structure of pyran, the cyclic form of glucose is also referred to as glucopyranose. In this ring, each carbon is linked to an hydroxyl side group with the exception of the fifth atom, which links to a sixth carbon atom outside the ring, forming a CH2OH group.
Space-filling model of glucose molecule
Isomers
Glucose has 4 optic centers which means that in theory glucose can have 15 optical stereoisomers. Only 7 of these are found in living organisms, and of these galactose (Gal) and mannose (Man) are the most important. These eight isomers (including glucose itself) are all diastereoisomers in relation to each other and all belong to the D-series.
An additional asymmetric center at C-1 (called the anomeric carbon atom) is created when glucose cyclizes and two ring structures, called anomers, can be formed — α-glucose and β-glucose. They differ structurally in the orientation of the hydroxyl group linked to C-1 in the ring. When D-glucose is drawn as a Haworth projection, the designation α means that the hydroxyl group attached to C-1 is below the plane of the ring, β means it is above. The α and β forms interconvert over a timescale of hours in aqueous solution, to a final stable ratio of α:β 36:64, in a process called mutarotation.
PRODUCTION
Natural
Glucose shifting from Fischer projection to Haworth projection
Commercial
Glucose is produced commercially via the enzymatic hydrolysis of starch. Many crops can be used as the source of starch Maize, rice, wheat, potato, cassava, arrowroot, and sago are all used in various parts of the world. In the United States, cornstarch (from maize) is used almost exclusively.
This enzymatic process has two stages. Over the course of 1-2 hours near 100 °C, these enzymes hydrolyze starch into smaller carbohydrates containing on average 5-10 glucose units each. Some variations on this process briefly heat the starch mixture to 130 °C or hotter one or more times. This heat treatment improves the solubility of starch in water, but deactivates the enzyme, and fresh enzyme must be added to the mixture after each heating.
In the second step, known as saccharification, the partially hydrolyzed starch is completely hydrolyzed to glucose using the glucoamylase enzyme from the fungus Aspergillus niger. Typical reaction conditions are pH 4.0–4.5, 60 °C, and a carbohydrate concentration of 30–35% by weight. Under these conditions, starch can be converted to glucose at 96% yield after 1–4 days. Still higher yields can be obtained using more dilute solutions, but this approach requires larger reactors and processing a greater volume of water, and is not generally economical. The resulting glucose solution is then purified by filtration and concentrated in a multiple-effect evaporator. Solid D-glucose is then produced by repeated crystallizations.
Function
We can speculate on the reasons why glucose, and not another monosaccharide such as fructose (Fru) , is so widely used. Glucose can form from formaldehyde under abiotic conditions, so it may well have been available to primitive biochemical systems. Probably more important to advanced life is the low tendency of glucose, by comparison to other hexose sugars, to non-specifically react with the amino groups of proteins. This reaction (glycation) reduces or destroys the function of many enzymes. The low rate of glycation is due to glucose's preference for the less reactive cyclic isomer. Nevertheless, many of the long-term complications of diabetes (e.g., blindness, kidney failure, and peripheral neuropathy) are probably due to the glycation of proteins or lipids. Glycosylation is another important type of reaction undergone by glucose.
As an energy source
Glucose is a ubiquitous fuel in biology. Carbohydrates are the human body's key source of energy, providing 4 kilocalories (17 kilojoules) of food energy per gram. Breakdown of carbohydrates (e.g. starch) yields mono- and disaccharides, most of which is glucose. Through glycolysis and later in the reactions of the Citric acid cycle (TCAC), glucose is oxidized to eventually form CO2 and water, yielding energy, mostly in the form of ATP. The insulin reaction, and other mechanisms, regulate the concentration of glucose in the blood. A high fasting blood sugar level is an indication of prediabetic and diabetic conditions.
As a precursor
Glucose is critical in the production of proteins and in lipid metabolism. Also, in plants and most animals, it is a precursor for vitamin C (ascorbic acid) production.
Glucose is used as a precursor for the synthesis of several important substances. Starch, cellulose, and glycogen ("animal starch") are common glucose polymers (polysaccharides). Lactose, the predominant sugar in milk, is a glucose-galactose disaccharide. In sucrose, another important disaccharide, glucose is joined to fructose.
Sources and absorption
All major dietary carbohydrates contain glucose, either as their only building block, as in starch and glycogen, or together with another monosaccharide, as in sucrose and lactose. In the lumen of the duodenum and small intestine the oligo- and polysaccharides are broken down to monosaccharides by the pancreatic and intestinal glycosidases. Glucose is then transported across the apical membrane of the enterocytes by SLC5A1 and later across their basal membrane by SLC2A2 (ref). Some of glucose goes directly to fuel brain cells and erythrocytes, while the rest makes its way to the liver and muscles, where it is stored as glycogen, and to fat cells, where it is stored as fat. Glycogen is the body's auxiliary energy source, tapped and converted back into glucose when there is needs for energy.
Solar Cola - a healthier alternative
|
||||||||||||||||||||||||||
|
This website is Copyright © 1999 & 2006 NJK. The bird logo and name Solar Navigator and Solar Cola are trademarks. All rights reserved. All other trademarks are hereby acknowledged. Max Energy Limited is an educational charity. |
||||||||||||||||||||||||||
|
BLUEBIRD ELECTRIC CAR MANUFACTURERS ELECTRIC CARS ELECTRIC CYCLES SOLAR CARS |
||||||||||||||||||||||||||