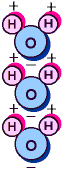|

What
are the physical and chemical properties of water that make it
so unique and necessary for living things? When you look at
water, taste and smell it - well, what could be more boring?
Pure water is virtually colorless and has no taste or smell. But
the hidden qualities of water make it a most interesting
subject.
|
|
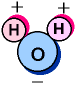
You
probably know water's chemical description is H2O.
As the diagram to the left shows, that is one atom of
oxygen bound to two atoms of hydrogen. The hydrogen
atoms are "attached" to one side of the
oxygen atom, resulting in a water molecule having a
positive charge on the side where the hydrogen atoms
are and a negative charge on the other side, where the
oxygen atom is. Since opposite electrical charges
attract, water molecules tend to attract each other,
making water kind of "sticky." As the
right-side diagram shows, the side with the hydrogen
atoms (positive charge) attracts the oxygen side
(negative charge) of a different water molecule. (If
the water molecule here looks familiar, remember that
everyone's favorite mouse is mostly water, too).
|
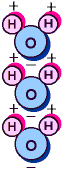
|
Water's
Chemical Properties
All
these water molecules attracting each other mean they tend to
clump together. This is why water drops are, in fact, drops! If
it wasn't for some of Earth's forces, such as gravity, a drop of
water would be ball shaped -- a perfect sphere. Even if it
doesn't form a perfect sphere on Earth, we should be happy water
is sticky.
Water
is called the "universal solvent" because it dissolves
more substances than any other liquid. This means that wherever
water goes, either through the ground or through our bodies, it
takes along valuable chemicals, minerals, and nutrients.
Pure
water has a neutral pH
of 7, which is neither acidic
nor basic.
Diagram
about pH
THE
PHYSICAL PROPERTIES of WATER:
-
Water
is unique in that it is the only natural substance that is
found in all three states -- liquid, solid (ice), and gas
(steam) -- at the temperatures normally found on Earth.
Earth's water is constantly interacting, changing, and in
movement.
-
Water
freezes at 32o Fahrenheit (F) and boils at 212o
F (at sea level, but 186.4° at 14,000 feet). In
fact, water's freezing and boiling points are the baseline
with which temperature is measured: 0o on the
Celsius scale is water's freezing point, and 100o
is water's boiling point. Water is unusual in that the solid
form, ice, is less dense than the liquid form, which is why
ice floats.
-
Water
has a high specific heat index. This means that water can
absorb a lot of heat before it begins to get hot. This is
why water is valuable to industries and in your car's
radiator as a coolant. The high specific heat index of water
also helps regulate the rate at which air changes
temperature, which is why the temperature change between
seasons is gradual rather than sudden, especially near the
oceans.
-
Water
has a very high surface tension. In other words, water is
sticky and elastic, and tends to clump together in drops
rather than spread out in a thin film. Surface tension is
responsible for capillary
action, which allows water (and its dissolved
substances) to move through the roots of plants and through
the tiny blood vessels in our bodies.
-
Here's
a quick rundown of some of water's properties:
-
Weight:
62.416 pounds per cubic foot at 32°F
-
Weight:
61.998 pounds per cubic foot at 100°F
-
Weight:
8.33 pounds/gallon, 0.036 pounds/cubic inch
-
Density:
1 gram per cubic centimeter (cc) at 39.2°F,
0.95865 gram per cc at 212°F
BY
THE WAY:
1 gallon = 4 quarts = 8 pints = 128 ounces = 231
cubic inches
1 liter = 0.2642 gallons = 1.0568 quart = 61.02
cubic inches
1 million gallons = 3.069 acre-feet = 133,685.64
cubic feet
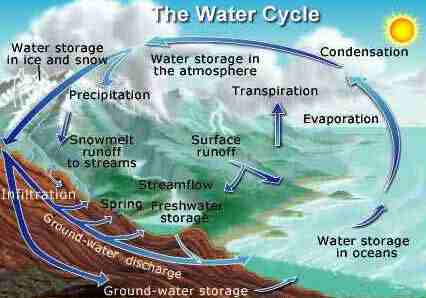
The
Water (or Hydrological) Cycle
The
hydrological cycle is the continuous movement of water between
the earth and the atmosphere. Water evaporates from
water and land surfaces and transpires from living cells.
This vapour circulates through the atmosphere, condensing to
form clouds and precipitating as rain or snow. When
water hits the earth's surface it either runs into streams and
ends up in oceans or lakes, or seeps into the soil. The
water that seeps into the soil is then either absorbed by the
roots of vegetation, or it sinks into the groundwater
reservoir.
Although
the balance of water on Earth remains fairly constant over
time, individual water molecules can come and go in a hurry.
The water in the apple you ate yesterday may have fallen as
rain half-way around the world last year or could have been
used 100 million years ago by Mama Dinosaur to give her baby a
bath.
CHEMICAL COMPOSITION
Water
is made up of hydrogen and oxygen. Two hydrogen atoms
are linked by a single chemical bond to one oxygen atom.
Its chemical formula is H2O.
The
water molecule is angular in shape, forming negative and
positive charges on opposite sides. This means that the
H2O molecule is highly polar. Because of this
high polarity H20 molecules form hydrogen bonds
which are very strong. This is when hydrogen atoms in
one water molecule are attracted to the non-bonding electron
pairs of the oxygen atom in another H20 molecule.
These strong hydrogen bonds mean that water has a very high
boiling point because it takes a lot more energy to overcome
them and release H2O molecules from the liquid into
the gaseous phase.
The
H2O molecules in ice are highly ordered, although
loosely structured. When ice melts this orderly
arrangement breaks and so H2O molecules can be
packed closer together. Therefore the liquid is denser
than the solid and this explains why ice floats on water.
One
of the most important properties of water is that it can
dissolve many other substances to form aqueous solutions.
This happens because of the H2O molecule's high
polarity. If, for example, an ionic compound such as
sodium chloride (NaCl) is added to water, the positively
charged Na atoms will be attracted to the negative end of the
H2O molecules, and the negatively charged Cl atoms
will be attracted to the positive end of the H20
molecules. Therefore the Na and Cl ions will be pulled
apart and hydrated, meaning they will be surrounded by H2O
molecules. This keeps the Na+ and Cl-
ions from recombining.
Water
can act as an acid or a base because it can dissociate to some
extent into H+ (hydrogen) ions which are acidic,
and OH- (hydroxyl) ions which are alkaline (basic).
Most
hydrogen atoms consist of only 1 proton, but the isotopes
deuterium and tritium have one and two neutrons in their
nuclei respectively. Deuterium oxide (D2O) is
called heavy water because it has a greater molecular
weight than H2O due to the extra neutron in the
deuterium nucleus. D2O can be produced
through electrolysis and fractional distillation of water.
It is used as a moderator of neutrons in nuclear power plants
and in biological research as an isotopic tracer.
WATER AS ICE :
Ice
occurs when water vapour or liquid water freeze. At
temperatures below 0°C (32F) water vapour becomes frost at
ground level and snowflakes (each one a single ice crystal) in
clouds, while liquid water becomes solid ice in the form of
river ice, sea ice, hail etc. Each H2O
molecule forms hydrogen bonds with four neighbouring
molecules, creating a tetrahedral shape. An ice crystal
is made up of rings of these tetrahedrons forming at various
angles to each other.
At
first sight ice seems brittle and shatters like glass when it
is struck. However it flows under low stresses over long
periods of time or under high stresses where pressure stops
the ice from splintering. This happens because the
layers of ice crystal can glide over and past each other
without the hydrogen bonds being broken. Flow is very
slow because the different crystals glide in different
directions and tend to interfere with one another.
Glaciers are an example of this flow.
Ice
is used as a refrigerating agent because it takes more energy
to melt it than most other substances, due to strong hydrogen
bonds. Melting ice remains at a constant 0°C (32F).
Because
ice is less dense than water at 0°C (32F) a mass of ice
occupies 9% more volume than an equal mass of water.
This is why when water in pipes freezes it can cause the pipes
to burst. When water enters tiny cracks in rocks and
freezes, the expansion creates great pressure that will split
the rocks, causing erosion.
Another
important point about ice being less dense than water is that
it floats. In rivers, lakes and oceans this means that
the ice traps the warmer water below, allowing for fish and
other creatures to survive the freezing temperatures of the
surface.
WATER as STEAM
Steam
is vapourised water and is an odourless, invisible gas.
It often looks white and cloudy because there are tiny water
droplets present. Steam is created in nature from
volcanic processes heating underground water and is released
through hot springs and geysers, for example. The
temperature at which water will boil depends on its pressure.
If pressure is reduced, the boiling point is also reduced.
If pressure is increased, more energy is required to allow the
liquid molecules to escape into the gaseous phase, and
therefore the temperature at which water boils also increases.
Modern
industrial society makes extensive use of steam power.
Virtually all the world's electricity is created through steam
power. Power plants heat water into steam which, under
pressure, drives turbines that produce electrical current.
Steam is also used in the manufacture of steel, aluminium,
copper and nickel, and the production of chemicals, the
refinement of petroleum and for cooking and heating in the
home.
WATER as a PRECIOUS RESOURCE
Most
of the earth's water is undrinkable. If a large bucket
of water were to represent the sea water on the planet, an egg
cup full would represent the amount of water locked in ice
caps and glaciers and a teaspoonful would be all that was
available as drinking water. Human use of natural
waters, especially freshwater resources such as rivers and
lakes, has steadily increased over the centuries. With
population growth and increasing use of water for agriculture,
industry and recreation, water is becoming an incredibly
valuable resource.
It
is not only the scarcity of water that is becoming an issue,
but also quality. Mineral fertilizers, pesticides and
herbicides have seeped into surface and subsurface waters
contaminating them beyond human consumption and disrupting
delicate ecosystems. Dumping of sewage and industrial
wastes and toxins pollute rivers and lakes and threaten
the world's most important resource. Will their be
enough water to accommodate the needs of future generations?

As
you know, the Earth is a watery place. Estimates vary, but
somewhere between 70 and 75 percent of the Earth's surface is
water-covered. But water also exists in the air as water vapor
and in the ground as soil moisture and in aquifers.
Thanks to the water
cycle (view a graphic
of the water cycle) our planet's water supply is constantly
moving from one place to another and from one form to another.
Things would get pretty stale without the water cycle!
When
you take a look at the water around you, you see water in
streams, rivers, and lakes. You see water sitting on the surface
of the earth. Naturally, this water is known as "surface
water." Your view of the water cycle might be that rainfall
fills up the rivers and lakes. But, how would you account for
the flow in rivers after weeks without rain? In fact, how would
you account for the water
flowing down this driveway on a day when it didn't
rain? The answer is that there is more to our water supply than
just surface water, there is also plenty of water beneath our
feet.
Even
though you may only notice water on the Earth's surface, there is
much more water stored in the ground than there is on the
surface. In fact, some of the water you see flowing in
rivers comes from seepage of ground water into river beds. Water
from precipitation continually seeps into the ground to recharge
the aquifers,
while at the same time water from underground aquifers
continually recharges rivers through seepage.
Humans
are happy this happens because people make use of both kinds of
water. In the United States in 2000, we used about 323 billion
gallons per day of surface
water and about 85 billion gallons per day of ground
water. In a way, that underestimates the importance of
ground water, since not only does ground water help keep our
rivers and lakes full, it also provides water for people in
places where visible water is scarce, such as in the desert
towns of the Western United States. Without ground water, people
would be sand-surfing in Palm Springs, Ca. instead of playing
golf!
Just
how much water is there on (and in) the Earth? Here are some
numbers you can think about:
-
The
total water supply of the world is 326 million cubic miles
(a cubic mile is an imaginary cube (a square box) measuring
one mile on each side). A cubic mile of water equals more
than one trillion gallons.
-
About
3,100 cubic miles of water, mostly in the form of water
vapor, is in the atmosphere at any one time. If it all fell
as precipitation at once, the Earth would be covered with
only about 1 inch of water.
-
The
48 contiguous United States receives a total volume of about
4 cubic miles of precipitation each day.
-
Each
day, 280 cubic miles of water evaporate
or transpire
into the atmosphere.
-
If
all of the world's water was poured on the United States, it
would cover the land to a depth of 90 miles.
Of
the freshwater on Earth, much more is stored in the ground than
is available in lakes
and rivers.
More than 2,000,000 cubic miles of fresh water is stored in the
Earth, most within one-half mile of the surface. Contrast that
with the 60,000 cubic miles of water stored as fresh water in
lakes, inland seas, and rivers. But, if you really want to find
fresh water, the most is stored in the 7,000,000 cubic miles of
water found in glaciers
and icecaps, mainly in the polar regions and in
Greenland.
FACTS
ABOUT WATER
-
Water
is odourless and tasteless. It has a bluish tint that can
be seen in very deep layers.
-
At
Standard Atmospheric Pressure (760mm of mercury) water has
a freezing point of 0°C (32F) and a boiling point of 100°C
(212F). Water is at its maximum density at 4°C
(39F).
-
Water
can remain a liquid even below its freezing point, up to
-25°C, if it is not disturbed and if the temperature does
not drop further and no particle or ice crystal is added
to it.
-
Water
is used in the metric system to define the gram.
-
Water
is the only substance that occurs at ordinary temperatures
in all three phases: liquid, gas and solid.
-
75%
of the earth's surface is covered by water.
-
The
oceans contain 97.5% of the earth's water, the land 2.4%,
and the atmosphere holds less than .001%
-
Only
1% of the earth's water is available for drinking; 2% is
frozen.
-
50-90%
of the weight of living organisms is made up of water.
Blood in animals and sap in plants is mostly water.
-
The
adult human body is composed of approximately 55 to 60%
water--the brain is composed of 70% water, as is skin,
blood is 82% water, and the lungs are nearly 90% water.
-
The
world average rainfall is 860 mm.
-
You
can survive about a month without food, but only 5-7 days
without water.
-
It
is possible to drink water today that was here in the
dinosaur age.
-
The
average urban home of 4.6 people uses 640 litres of water
per day.
-
A
dripping tap can waste as much as 60 litres per day or 1
800 litres per month.
-
A
leaking toilet can waste up to 100 000 litres of water per
year, enough to take three full baths every day.
-
It
takes about 2.5 litres of water to cook pasta and about 5
litres to clean the pot.
-
The
average bath holds between 150 and 200 litres of water
when filled to the brim.
-
A
toilet is the biggest user of indoor water. On
average, it uses 11 litres of water when flushed.
     
     
     
INTERNATIONAL
WATER
The
boundaries between countries are political constructs which
often do not take into consideration the distribution of
natural resources. For example, rivers have been used as
international boundaries for centuries and, although rivers
may form logical barriers between people, they by definition
fall in the centre of drainage basins.
Political
boundaries therefore divide water courses, river basins and
groundwater aquifers. Where ever this happens the water
course, river basin or aquifer becomes international.
This complicates the management of water resources enormously.
A good example of this is in Southern Africa where all rivers
of size are shared between at least two countries and every
country has at least 1 international river, with Mozambique
has 9.
International
water resources management is a complex process. Whilst
it may lead to tension and stress between countries, it also
provides opportunities for co-operation to maximize the mutual
benefits of the resources. International water resources
management requires both political and technical process which
usually needs a legal basis through which to function.
In many instances the institution which manages the process is
a river or basin Commission. The establishment of all of
the necessary elements often requires many years of
negotiation and planning and is subject to many disruptions
and hurdles which often have nothing to do with water.
Countries
regulate their relationships with regards to shared water
courses through treaties, protocols, agreements and other
legal instruments. These usually address such issues as
water quality, water utilisation and abstraction, the
construction of hydraulic structures such as dams and weirs
for irrigation, hydro-power generation and flood management,
notification and conflict resolution.
Take
a True/False
quiz about water properties. Some of the answers may
surprise you!
WATER
LINKS:
The
Elizabeth Swan is
a zero carbon ship powered by solar panels and wind turbines.
This
robot ship may be manned for the Kulo
Luna environmental adventure story.
|