|
OXYGEN
|
|
HOME | BIOLOGY | FILMS | GEOGRAPHY | HISTORY | INDEX | INVESTORS | MUSIC | SOLAR BOATS | SPORT |
|
Oxygen is a chemical element with the chemical symbol O and atomic number 8. On Earth, it is usually covalently or ionically bonded to other elements.
Unbound oxygen (also called molecular dioxygen, O2, a diatomic molecule) first appeared in significant quantities on Earth during the Paleoproterozoic era (between 2.5 billion years ago and 1.6 billion years ago) as a product of the metabolic action of early anaerobes (archaea and bacteria). The presence of large amounts of free oxygen may have driven most of the organisms then living to extinction. The atmospheric abundance of free oxygen in later geological epochs and up to the present has been largely driven by photosynthetic organisms; roughly three quarters of the free element being produced by algae in the oceans, and one quarter from terrestrial plants.
Characteristics
Oxygen is a major component of air, produced by plants during photosynthesis, and is necessary for aerobic respiration in animals. The word oxygen derives from two roots in Greek, οξυς (oxys) (acid, sharp) and -γενης (-genēs) (born of). In the early 18th century, Antoine Lavoisier coined the name oxygen from the Greek roots mentioned above because he erroneously thought that it was a constituent of all acids. (The definition of acid has since been revised).
At standard temperature and pressure, oxygen exists as a diatomic molecule with the formula O2, in which the two oxygen atoms are doubly bonded to each other. In its most stable form, oxygen exists as a diradical (triplet oxygen) with two unpaired electrons in molecular orbitals of the O2 molecule. Though unpaired electrons are commonly associated with high reactivity in chemical compounds, triplet oxygen is relatively (and fortunately) unreactive by comparison with most radicals.
Singlet oxygen, a name given to several higher energy species of molecular oxygen in which all the electron spins are paired, is much more reactive towards common organic molecules. In nature, singlet oxygen is commonly formed from water during photosynthesis, using the energy of sunlight. It is also produced by the immune system as a source of active oxygen. Carotenoids in photosynthetic organisms and possibly also in animals, play a major role in absorbing energy from singlet oxygen and converting it to the unexcited ground state, before it can cause harm to tissues.
Liquid O2 and solid O2 are clear substances with a light sky-blue color. In normal triplet form they are paramagnetic due to the spin magnetic moments of the unpaired electrons in the molecule, and the negative exchange energy between neighbouring O2 molecules. Liquid oxygen is attracted to a magnet to a sufficient extent that a bridge of liquid oxygen may be supported against its own weight between the poles of a powerful magnet, in laboratory demonstrations. Liquid O2 is usually obtained by the fractional distillation of liquid air.
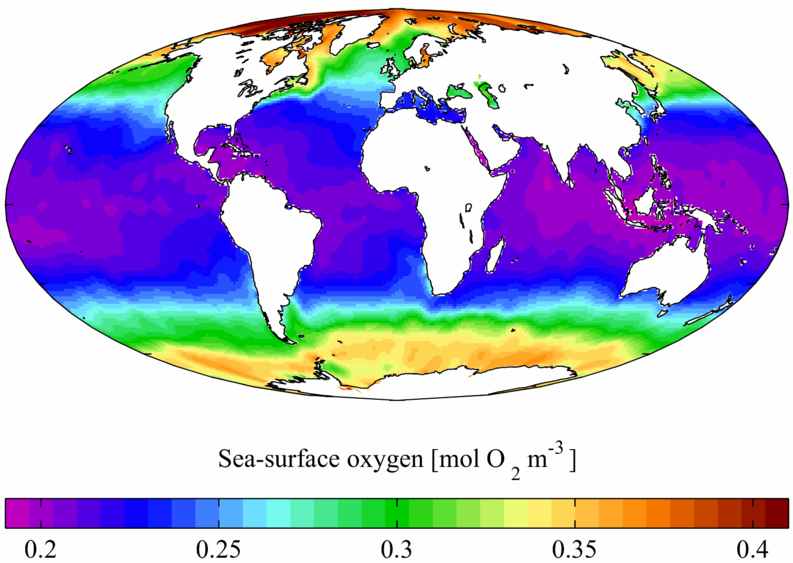
Oxygen is slightly soluble in water, but naturally occuring disolved amounts support all ocean animal life (see below).
O2 has a bond length of 121 pm and a bond energy of 498 kJ/mol.
Occurence
Oxygen is the most common component of the Earth's crust (49% by mass), the second most common component of the Earth as a whole (28% by mass), the most common component of the world's oceans (86% by mass), and the second most common component of the Earth's atmosphere (20.947% by volume), second to nitrogen.
Elemental oxygen occurs not only in the atmosphere, but also as solution in the world's water bodies. At 25° C under 1 atm of air, a litre of water will dissolve about 6.04 cc (8.63 mg, 0.270 mmol) of oxygen, whereas sea water will dissolve about 4.9 cc (7.0 mg, 0.22 mmol). At 0° C the solubilities increase to 10.29 cc (14.7 mg, 0.460 mmol) for water and 8.0 cc (11.4 mg, 0.36 mmol) for sea water. This difference has important implications for ocean life, as polar oceans support a much higher density of life due to their oxygen content.
LINKS and REFERENCE
A taste for adventure capitalists
Solar Cola - a healthier alternative
|
|
This
website
is Copyright © 1999 & 2006 NJK. The bird |
|
AUTOMOTIVE | BLUEBIRD | ELECTRIC CARS | ELECTRIC CYCLES | SOLAR CARS |